This group of medicines are rarely used today due to the side effect profile including weight gain, peripheral oedema and increase risk of heart failure and fractures.
- JANUMET is indicated as an adjunct to healthy eating and physical activity to
improve glycaemic management in adults with type 2 diabetes mellitus when
treatment with both sitagliptin and metformin is appropriate.
For the latest PBS indications for JANUMET please see
https://www.pbs.gov.au/medicine/item/10089B-10090C-9449H-9450J-9451K
Life threatening lactic acidosis can occur due to accumulation of metformin. The main risk factor is renal impairment, other risk factors include old age associated with reduced renal function and high doses of metformin above 2g per day.
- The dosage of diabetes therapy with JANUMET should be individualised based on a person’s current regimen, effectiveness and tolerability while not exceeding the maximum recommended daily dose of 100 mg sitagliptin and 2000 mg metformin. (Person centred care)
- JANUMET should generally be given twice daily with meals, with gradual dose escalation, to reduce the gastrointestinal (GI) side effects due to metformin.
- As initial therapy:
- For individuals with type 2 diabetes mellitus, whose hyperglycaemia is inadequately managed with healthy eating and physical activity, when dual therapy is appropriate, the recommended total daily starting dose of JANUMET is 100 mg sitagliptin and 1000 mg metformin hydrochloride. Individuals with inadequate glycaemic management on this dose should have their metformin dose increased up to a maximum of 100 mg sitagliptin/2000 mg metformin hydrochloride daily.
- For individuals inadequately managed on sitagliptin monotherapy: For those whose diabetes is inadequately on sitagliptin alone, the recommended starting dose of JANUMET is 100 mg sitagliptin and 1000 mg metformin hydrochloride daily. Individuals may be titrated up to 100 mg sitagliptin/2000 mg metformin hydrochloride daily to achieve healthy glycaemia. Individuals taking sitagliptin monotherapy dose-adjusted for renal impairment should not be switched to JANUMET.
- For individuals, whose diabetes is inadequately managed on metformin monotherapy: For those whose diabetes is not adequately managed on metformin alone, the usual starting dose of JANUMET should provide sitagliptin 100 mg total daily dose plus the dose of metformin already being taken.
- For individuals switching from sitagliptin co-administered with metformin: For individuals switching from sitagliptin co-administered with metformin, JANUMET may be initiated at the dose of sitagliptin, and metformin already being taken.
- For individuals who diabetes is inadequately managed on dual combination therapy with metformin and a sulfonylurea: The usual starting dose of JANUMET should provide sitagliptin 100 mg total daily dose and the dose of metformin already being taken. Individuals may require lower sulfonylurea doses to reduce the risk of sulfonylurea-induced hypoglycaemia.
Note: Diabetes MedsCheck with counselling on managing hypoglycaemia and referral for blood glucose monitoring.
- For individuals, whose diabetes is inadequately managed on dual combination therapy with metformin and insulin: The usual starting dose of JANUMET should provide 100 mg total daily dose of sitagliptin. In determining the starting dose of the metformin component, an individual’s level of glycaemia and current dose of metformin should be considered. Individuals currently on or initiating insulin therapy may require lower doses of insulin to reduce the risk of hypoglycaemia.
Note: Diabetes MedsCheck with counselling on managing hypoglycaemia and referral for blood glucose monitoring - Renal impairment: No dose adjustment is needed in mild renal impairment (estimated glomerular filtration rate [eGFR] ≥ 60 mL/min/1.73 m2). An eGFR should be assessed before initiation of treatment with JANUMET and at least annually thereafter. In those at increased risk of further progression of renal impairment and in the elderly, renal function should be assessed more frequently, e.g. every 3-6 months.
- The maximum daily dose of metformin should preferably be divided into 2-3 daily doses.
- Factors that may increase risk of lactic acidosis should be reviewed before considering initiation of metformin in those with eGFR < 60 mL/min/1.73 m2
- JANUMET is contraindicated with eGFR < 30 mL/min/1.73 m2
- JANUMET is not recommended in those with an eGFR ≥ 30 mL/min/1.73 m2 and <45 mL/min/1.73 m2 because these individuals require a lower dosage of sitagliptin than what is available in the fixed combination JANUMET product.

- Hepatic impairment
- Sitagliptin phosphate monohydrate: In individuals with moderate hepatic impairment (Child-Pugh score 7 to 9), mean AUC and Cmax of sitagliptin increased approximately 21% and 13%, respectively, compared to healthy matched controls following administration of a single 100 mg dose of sitagliptin. These differences are not considered to be clinically meaningful. There is no clinical experience in individual with severe hepatic impairment (Child-Pugh score > 9). However, because sitagliptin is primarily renally eliminated, severe hepatic impairment is not expected to affect the pharmacokinetics of sitagliptin. - Metformin hydrochloride: No pharmacokinetic studies of metformin have been conducted in those with hepatic impairment. Impaired hepatic function has been associated with some cases of lactic acidosis in those taking metformin, however.
As metformin and sitagliptin are excreted by the kidney, JANUMET should be used with caution as age increases. Monitoring of renal function is necessary to aid in prevention of metformin-associated lactic acidosis, particularly in the elderly.
JANUMET are not currently indicated for use in children below 18 years.
- Severe renal impairment (eGFR < 30 mL/min/1.73 m2)
- Known hypersensitivity to sitagliptin phosphate monohydrate, metformin hydrochloride or any other component of JANUMET
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
- JANUMET should be temporarily discontinued when undergoing radiological studies involving intravascular administration of iodinated contrast materials, because the use of such products may result in acute alteration of renal function.
- Sitagliptin
- During post marketing experience the following adverse reactions have been reported with use of s: serious hypersensitivity reactions, including anaphylaxis and angioedema. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency. If a serious hypersensitivity reaction to sitagliptin is suspected, discontinue JANUMET, assess for other potential causes for the event, and institute alternative treatment for diabetes.
Note: Diabetes MedsCheck with counseling on side effect profile and referral to healthcare team as required.
Pancreatitis: During post-marketing experience, there have been spontaneously reported adverse reactions of acute pancreatitis. Individuals should be informed of the characteristic symptom of acute pancreatitis: persistent, severe abdominal pain. If pancreatitis is suspected, JANUMET should be discontinued. Note: Diabetes MedsCheck with counseling on side effect profile and referral to healthcare team as required. - Bullous pemphigoid: post-marketing cases of bullous pemphigoid requiring hospitalisation have been reported with DPP4 inhibitor use. In reported cases, individuals typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell individuals to report development of blisters or erosions while receiving JANUMET. If bullous pemphigoid is suspected, JANUMET should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment. Note: Diabetes MedsCheck with counselling on side effect profile and referral to healthcare team as required. Arthralgia: There have been post marketing reports of joint pain, which may be severe, in those taking DPP4 inhibitors. Onset of symptoms following initiation of treatment may be rapid or may occur after longer periods. Discontinuation of therapy should be considered in individuals who present with or experience an exacerbation of joint symptoms during treatment with Sitagliptin. Note: Diabetes MedsCheck with counseling on side effect profile and referral to healthcare team as required.
- General - JANUMET XR should not be used in individuals with type 1 diabetes mellitus.
- Metformin
-Lactic acidosis: Lactic acidosis is a rare, but serious metabolic complication that can occur due to metformin accumulation during treatment with metformin. When it occurs, it is fatal in approximately 50% of cases. Lactic acidosis is a medical emergency and must be treated in hospital immediately. The risk of lactic acidosis increases with the degree of renal dysfunction. Reported cases of lactic acidosis in individuals on metformin have occurred primarily in those with diabetes with significant renal insufficiency, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Special caution should be taken in the elderly due to the decrease of renal function with age.
Note: Diabetes MedsCheck for referral to healthcare team for education on lactic acidosis.
-Surgery: Metformin must be discontinued at the time of surgery under general, spinal or epidural anaesthesia. Therapy may be restarted no earlier than 48 hours following surgery or resumption of oral nutrition and provided that renal function has been re-evaluated and found to be stable.
- Monitoring of renal function: Renal function should be confirmed before initiation of JANUMET therapy, and then at least once a year in those with normal renal function and at least two to four times a year if serum creatinine levels are at or above the upper limit of normal and in elderly individuals.
Decreased renal function in the elderly is frequent and asymptomatic. Special caution should be exercised in situations where renal function may become impaired, for example when initiating antihypertensive or diuretic therapy or when starting treatment with a nonsteroidal anti-inflammatory drug (NSAID).
Note: Diabetes MedsCheck with referral for annual cycle of care. - Alcohol Intake: Alcohol is known to potentiate the effect of metformin on lactate metabolism. Individuals, therefore, should be warned against excessive alcohol intake while receiving JANUMET XR.
- Metformin
-Gastrointestinal disorders: Gastrointestinal symptoms such as nausea, vomiting, diarrhoea, abdominal pain, and loss of appetite are very common (>10%): these occur most frequently during initiation of therapy and resolve spontaneously in most cases. To prevent these gastrointestinal symptoms, it is recommended that this medicinal product be taken in 2 or 3 daily doses. A slow increase of the dose may also improve gastrointestinal tolerability.
-Metabolism and nutrition disorders: Lactic acidosis is a very rare (<0.01%) but serious metabolic complication that can occur due to metformin accumulation during treatment with metformin. The onset of lactic acidosis is often subtle and accompanied only by non-specific symptoms such as malaise, myalgia, respiratory distress, increasing somnolence and non-specific abdominal distress. There may be associated hypothermia, hypotension and resistant bradyarrhythmias with more marked acidosis.
Note: Diabetes MedsCheck with referral to healthcare team for education on lactic acidosis.
-Hepatobiliary disorders: Very rare: liver function test abnormalities or hepatitis requiring treatment discontinuation.
-Skin and subcutaneous tissue disorders: Skin reactions such as erythema, pruritus and urticaria have been reported but the incidence is very rare (<0.01%).
-Nervous system disorders
-Taste disturbance (3 %) is common.
-Vitamin B12 Levels: In controlled, 29-week clinical trials of immediate release metformin, a decrease to subnormal levels of previously normal serum Vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex is, however, very rarely associated with anaemia and appears to be rapidly reversible with discontinuation of metformin or Vitamin B12 supplementation. Measurement of haematologic parameters on an annual basis is advised in patients on JANUMET XR and any apparent abnormalities should be appropriately investigated and managed. Certain individuals (those with inadequate Vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal Vitamin B12 levels). See interventions for further information. - Sitagliptin
- During post marketing experience the following adverse reactions have been reported with use of s: serious hypersensitivity reactions, including anaphylaxis and angioedema. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency. If a serious hypersensitivity reaction to sitagliptin is suspected, discontinue JANUMET, assess for other potential causes for the event, and institute alternative treatment for diabetes.
Note: Diabetes MedsCheck with counseling on side effect profile and referral to healthcare team as required.
Pancreatitis: During post marketing experience, there have been spontaneously reported adverse reactions of acute pancreatitis. Individuals should be informed of the characteristic symptom of acute pancreatitis: persistent, severe abdominal pain. If pancreatitis is suspected, JANUMET should be discontinued. Note: Diabetes MedsCheck with counseling on side effect profile and referral to healthcare team as required.
- Bullous pemphigoid: post-marketing cases of bullous pemphigoid requiring hospitalisation have been reported with DPP4 inhibitor use. In reported cases, individuals typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell individuals to report development of blisters or erosions while receiving JANUMET. If bullous pemphigoid is suspected, JANUMET should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
Note: Diabetes MedsCheck with counselling on side effect profile and referral to healthcare team as required.
Arthralgia: There have been post marketing reports of joint pain, which may be severe, in those taking DPP4 inhibitors. Onset of symptoms following initiation of treatment may be rapid or may occur after longer periods. Discontinuation of therapy should be considered in individuals who present with or experience an exacerbation of joint symptoms during treatment with Sitagliptin.
Note: Diabetes MedsCheck with counseling on side effect profile and referral to healthcare team as required.
Liraglutide
- Saxenda is a solution for injection in a pre-filled pen. One mL contains 6 mg salt-free anhydrous liraglutide. One pre-filled pen contains 18 mg liraglutide in 3 mL
- Therapeutic indications:
- Please note Saxenda is NOT PBS or TGA approved for treatment of type 2 diabetes. Instead, it is TGA (not PBS listed) approved as a product for weight management.
- Saxenda is indicated as an adjunct to a reduced calorie eating plan and increased physical activity for weight management in adults with an initial Body Mass Index (BMI) of ≥ 30 kg/m (obese) or ≥ 27 kg/m to < 30 kg/m (overweight) in the presence of at least one weight related comorbidity, such as dysglycaemia (pre-diabetes and type 2 diabetes mellitus), hypertension, dyslipidaemia, or obstructive sleep apnoea.
KnowDiabetes Diabetes Prevention Program
Diabetes reversal should be a goal in the management of type 2 diabetes
The KnowDiabetes Diabetes Prevention Plan draws on the latest research in diabetes prevention to assist you or your family members to understand how to reduce risk.
One on one personalised support!
1
Understand key risk factors
2
Learn how to minimise risk
3
Simple guided steps towards health & wellbeing
4
Be healthier and reduce your risk

Program Designed by
Dr. Alan Barclay
Accredited Practising Dietitian
Dr. Alan Barclay is an accredited practicing dietitian and nutritionist with over 25 years of experience in clinical dietetics, public health and academia. He completed a PhD at the University of Sydney on the association between carbohydrate (starches and sugars) and the risk of developing lifestyle-related diseases like type 2 diabetes. He is an honorary associate at the University of Sydney and is a consultant to the Glycemic Index Foundation. He was Head of Research at the Australian Diabetes Council (Diabetes Australia (NSW)) from 1998-2014.
Alan appears frequently in newspapers, magazines, radio and television news. He has authored or co-authored 5 books including Reversing Diabetes.
About the program
by Dr. Alan Barclay
Our personalised program works with you to achieve a healthy weight and body composition. It’s about achieving healthy body fat and muscle ratios which support health and reduces risk. Helping you to feel better and look better
How does it work?
The KnowDiabetes Type 2 Prevention Program is now available through select community pharmacies. Simply locate a pharmacy provider and book a time to find out how the program may assist you or a family member.
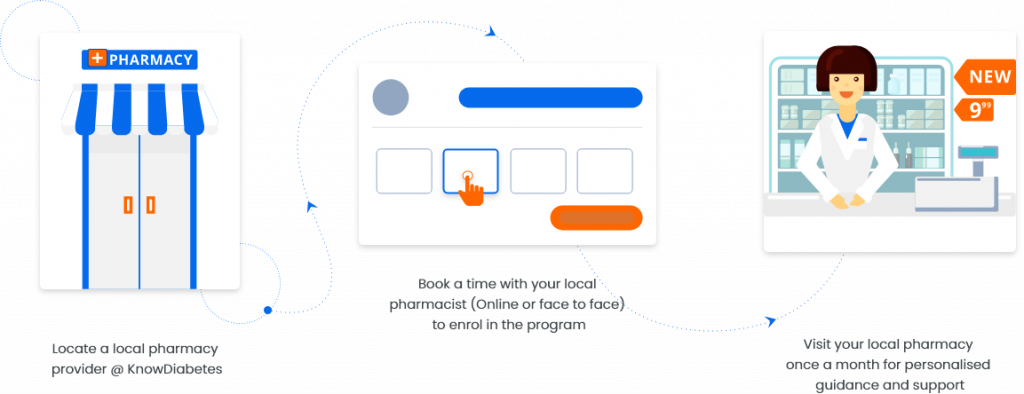
On signing up, you will receive appointment confirmation via e-mail to your account at your appointment time to start your session.

© 2024 Movely. All Rights Reserved.
KnowDiabetes Diabetes Prevention Program
Diabetes reversal should be a goal
in the management of type 2 diabetes
The KnowDiabetes Diabetes Prevention Plan draws on the latest research in diabetes prevention to help prevent people with IFG/IGT from developing type2 diabetes
One on one personalised support!
1
Easy to use online portal
2
Learn how to minimise risk
3
Simple guided steps towards health & wellbeing
4
Be healthier and reduce your risk
About the program
by Dr. Alan Barclay
Our personalised program works with you to achieve a healthy weight and body composition. It’s about achieving healthy body fat and muscle ratios which support health and reduces risk. Helping you to feel better and look better
How does it work?
The KnowDiabetes Type 2 Prevention Program is now available through select community pharmacies. Simply locate a pharmacy provider and book a time to find out how the program may assist you or a family member.

On signing up, you will receive appointment confirmation via e-mail to your account at your appointment time to start your session.

© 2024 Movely. All Rights Reserved.
For more detailed information on this product please consult the source product information.
https://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Saxenda%20pi3.p
- The results of a definitive bioequivalence study in healthy subjects demonstrated that the JANUMET XR (sitagliptin phosphate monohydrate/metformin hydrochloride) 50 mg/500 mg and 50 mg/1000 mg combination tablets are bioequivalent to coadministration of corresponding doses of sitagliptin phosphate monohydrate (JANUVIA®) and metformin hydrochloride as individual tablets. Because bioequivalence is demonstrated at the lowest and highest combination tablet dose strengths available, bioequivalence is conferred to the (sitagliptin/metformin) 50 mg/850 mg fixed dose combination (FDC) tablet.
- After administration of JANUMET XR tablets with a high-fat breakfast, the AUC for sitagliptin was not altered. The mean Cmax was decreased by 17%, although the median Tmax was unchanged relative to the fasted state. After administration of JANUMET XR with a high-fat breakfast, the AUC for metformin increased 62%, the Cmax for metformin decreased by 9%, and the median Tmax for metformin occurred 2 hours later relative to the fasted state.
- Sitagliptin phosphate monohydrate: The mean volume of distribution at steady state following a single 100 mg intravenous dose of sitagliptin to healthy subjects is approximately 198 litres. The fraction of sitagliptin reversibly bound to plasma proteins is low (38%).
- Metformin hydrochloride: The apparent volume of distribution (V/F) of metformin following single oral doses of metformin hydrochloride tablets 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins, in contrast to sulfonylureas, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metformin hydrochloride tablets, steady state plasma concentrations of metformin are reached within 24-48 hours and are generally < 1 microgram/mL.
- Sitagliptin phosphate monohydrate: Sitagliptin is primarily eliminated unchanged in urine, and metabolism is a minor pathway. Approximately 79% of sitagliptin is excreted unchanged in the urine.
- Metformin hydrochloride: Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion.
- Sitagliptin phosphate monohydrate: Following administration of an oral [14C] sitagliptin dose to healthy subjects, approximately 100% of the administered radioactivity was eliminated in faeces (13%) or urine (87%) within one week of dosing. The apparent terminal t1/2 following a 100 mg oral dose of sitagliptin was approximately 12.4 hours and renal clearance was approximately 350 mL/min. Elimination of sitagliptin occurs primarily via renal excretion and involves active tubular secretion.
- Metformin hydrochloride: Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
For further information on JANUMET XR please see
https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-03939-3&d=202106081016933
Sitagliptin phosphate monohydrate/metformin hydrochloride
- JANUMET XR is available for oral administration as film-coated tablets containing sitagliptin phosphate monohydrate equivalent to 50 mg sitagliptin as free base and either 500 mg metformin hydrochloride modified release (JANUMET XR 50 mg/500 mg*), or 1000 mg metformin hydrochloride modified release (JANUMET XR 50 mg/1000 mg).
- Additionally, JANUMET XR is available for oral administration as tablets containing sitagliptin phosphate monohydrate equivalent to 100 mg sitagliptin as free base and 1000 mg metformin hydrochloride modified release (JANUMET XR 100 mg/1000 mg).
- Saxenda is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial Body Mass Index (BMI) of ≥ 30 kg/m2 (obese) or≥ 27 kg/m2 to < 30 kg/m2 (overweight) in the presence of at least one weight related comorbidity, such as dysglycaemia (pre-diabetes and type 2 diabetes mellitus), hypertension, dyslipidaemia, or obstructive sleep apnoea.
- Treatment with Saxenda should be discontinued after 12 weeks on the 3.0 mg/day dose if a patient has not lost at least 5% of their initial body weight.
- Long term use should be informed by the following: Therapeutic Goods Administration AusPAR VICTOZA, SAXENDA liraglutide Novo Nordisk Pharmaceuticals Pty. Ltd. - PM-2016-003931- 1-5 - FINAL 29 April 2019 -Page 8 of 51 Long term safety data are limited. Adverse reactions that are uncommon (frequency < 1/100) and/or are associated with prolonged use (> 12 months) might not have been identified in the clinical development program. (refer Clinical Trials).
- The starting dose is 0.6 mg once daily. The dose should be increased to 3.0 mg daily in increments of 0.6 mg with at least one-week intervals to improve gastrointestinal side effects. If escalation to the next dose step is not tolerated for two consecutive weeks, consider discontinuing treatment. Daily doses higher than 3.0 mg are not recommended.
- The need for continued treatment should be re-evaluated whenever a new prescription is written and at least yearly.
- Elderly (> 65 years old): No dose adjustment is required based on age. Caution should be exercised for individuals aged 65-74 years. It is not recommended that Saxenda be used for those over the age of 75 due to the lack of data.
- Hepatic impairment: Saxenda is not recommended in hepatic impairment.
- Renal impairment: No dose adjustment is required for those with mild or moderate renal impairment. Saxenda is currently not recommended for use in severe renal impairment including end-stage renal disease.
- Saxenda and Victoza both contain the same active ingredient, liraglutide, and therefore should not be used together. Saxenda should also not be used in combination with another GLP-1 receptor agonist.
Hypersensitivity to liraglutide or any active ingredient.
- Saxenda must not be used as a substitute for insulin. Saxenda has not been studied in individuals taking insulin. Therefore, Saxenda and insulin should not be used together. However, if Saxenda is prescribed with insulin or insulin secretagogues (such as sulfonylureas), consider reducing the dose of insulin or sulfonylureas to reduce the risk of hypoglycaemia. Note: MedsCheck to check for clinical interactions with referral to healthcare team, provide hypoglycaemia information, assess side effect profile, refer for blood glucose monitoring.
- Saxenda is not indicated for the treatment of type 2 diabetes mellitus.
- Saxenda is not indicated in individuals with obesity secondary to endocrinological or eating disorders or in conjunction with treatment with medicinal products that may cause weight gain.
- Saxenda is not recommended in combination with other medicinal products intended for weight loss, including prescription medicines, over-the-counter medicines, and complementary medicines or herbal preparations. Note: MedsCheck to check for clinical interactions-refer to heath care professionals if appropriate.
- Cardiovascular events: An increase in heart rate with Saxenda was observed in clinical trials. For those who experience a sustained increase in resting heart rate, Saxenda should be discontinued.
- Dehydration, renal impairment, and acute renal failure: Individuals treated with Saxenda should be advised of the potential risk of dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion. In those treated with GLP-1 receptor agonists, including liraglutide, there have been reports of acute renal injury/failure and worsening of chronic renal failure. Note: MedsCheck to counsel on possible side effects and complications, referral to appropriate health care professional for review.
- Pancreatitis: Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. After initiation of Saxenda, observe carefully for signs and symptoms of pancreatitis. Do not restart Saxenda if pancreatitis is confirmed. Note: MedsCheck to counsel on side effect profile and how to manage if it happens.
- Gastrointestinal disease: Saxenda has not been studied in individuals with any form of severe gastrointestinal disease, including gastroparesis. Note: MedsCheck for side effect profile, prevention of complications, referral to appropriate allied health care team.
- Hypoglycaemia with concomitant use of diabetes therapy: The risk of serious hypoglycaemia is increased when Saxenda is used in combination with insulin secretagogues (e.g., sulfonylureas) in individuals with type 2 diabetes. The risk of hypoglycaemia can be lowered by a reduction in the dose of sulfonylurea.
- Thyroid disease: Saxenda should be used with caution in patients with thyroid disease.
- Suicide behaviour and ideation: Individuals treated with Saxenda should be monitored for the emergence of depression, suicide thoughts or behaviour, or any unusual changes in mood or behaviour. Discontinue Saxenda in those that experience suicidal thoughts or behaviours or who develop other symptoms of depression referral to appropriate allied health Note: MedsCheck for referral to appropriate health care team.
- Gastrointestinal adverse reactions: Nausea, diarrhoea, constipation, vomiting, dyspepsia, abdominal pain, dry mouth, gastritis, gastroesophageal reflux, flatulence, eructation, and abdominal distension.
Note: MedsCheck, referral to allied health professional due to side effect profile. - Hypoglycaemia in individuals with type 2 diabetes
- Thyroid C-cell tumours: Liraglutide causes thyroid C-cell tumours at clinically relevant exposures in both genders of rats and mice. It is unknown whether Saxenda causes thyroid C-cell tumours, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumours has not been determined.
Note: MedsCheck to ensure relevant referral pathways for screening. - Malignancies: In the clinical development program for weight loss, there was no imbalance for all neoplasms, combined. However, when subgroup analyses were done by individual types of cancer, imbalances were identified, including invasive breast cancer in women and colorectal neoplasms (mainly adenomas). For further information please see the full product information.
- Injection site reactions: Injection site reactions have been reported in individuals treated with Saxenda. These reactions have usually been mild and transient.
Note: MedsCheck, referral to allied health professional due to side effect profile. - Suicidal behaviour and ideation:
Note: MedsCheck side effect profile referral to GP - Cardiac disorders: Increased heart rate.</li.
- Gastrointestinal disorders. Acute pancreatitis, haemorrhagic and necrotising pancreatitis.
Note: MedsCheck, inform about side effect profile, what symptoms to be aware of for and what to do should any of these side effects occur.
The absorption of liraglutide following subcutaneous administration is slow, reaching maximum concentration approximately 11 hours post dosing.
The mean apparent volume of distribution after subcutaneous administration of a liraglutide 3.0 mg is 20-25 L (for a person weighing approximately100 kg). The mean volume of distribution after intravenous administration of liraglutide is 0.07 L/kg. Liraglutide is extensively bound to plasma protein (> 98%).
During the 24 hours following administration of a single liraglutide dose to healthy individuals, the major component in plasma was intact liraglutide. Two minor plasma metabolites were also detected.
Liraglutide is endogenously metabolised in a similar manner to large proteins without a specific organ as major route of elimination. The elimination half-life is approximately 13 hours.
For more detailed information on this product please consult the source product information.
https://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Saxenda%20pi3.pdf
Liraglutide
- Saxenda is a solution for injection in a pre-filled pen. One mL contains 6 mg salt-free anhydrous liraglutide. One pre-filled pen contains 18 mg liraglutide in 3 mL
- Therapeutic indications:
- Please note Saxenda is NOT PBS or TGA approved for treatment of type 2 diabetes. Instead, it is TGA (not PBS listed) approved as a product for weight management.
- Saxenda is indicated as an adjunct to a reduced calorie eating plan and increased physical activity for weight management in adults with an initial Body Mass Index (BMI) of ≥ 30 kg/m (obese) or ≥ 27 kg/m to < 30 kg/m (overweight) in the presence of at least one weight related comorbidity, such as dysglycaemia (pre-diabetes and type 2 diabetes mellitus), hypertension, dyslipidaemia, or obstructive sleep apnoea.
- Saxenda is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial Body Mass Index (BMI) of ≥ 30 kg/m2 (obese) or≥ 27 kg/m2 to < 30 kg/m2 (overweight) in the presence of at least one weight related comorbidity, such as dysglycaemia (pre-diabetes and type 2 diabetes mellitus), hypertension, dyslipidaemia, or obstructive sleep apnoea.
- Treatment with Saxenda should be discontinued after 12 weeks on the 3.0 mg/day dose if a patient has not lost at least 5% of their initial body weight.
- Long term use should be informed by the following: Therapeutic Goods Administration AusPAR VICTOZA, SAXENDA liraglutide Novo Nordisk Pharmaceuticals Pty. Ltd. - PM-2016-003931- 1-5 - FINAL 29 April 2019 -Page 8 of 51 Long term safety data are limited. Adverse reactions that are uncommon (frequency < 1/100) and/or are associated with prolonged use (> 12 months) might not have been identified in the clinical development program. (refer Clinical Trials).
- The starting dose is 0.6 mg once daily. The dose should be increased to 3.0 mg daily in increments of 0.6 mg with at least one-week intervals to improve gastrointestinal side effects. If escalation to the next dose step is not tolerated for two consecutive weeks, consider discontinuing treatment. Daily doses higher than 3.0 mg are not recommended.
- The need for continued treatment should be re-evaluated whenever a new prescription is written and at least yearly.
- Elderly (> 65 years old): No dose adjustment is required based on age. Caution should be exercised for individuals aged 65-74 years. It is not recommended that Saxenda be used for those over the age of 75 due to the lack of data.
- Hepatic impairment: Saxenda is not recommended in hepatic impairment.
- Renal impairment: No dose adjustment is required for those with mild or moderate renal impairment. Saxenda is currently not recommended for use in severe renal impairment including end-stage renal disease.
- Saxenda and Victoza both contain the same active ingredient, liraglutide, and therefore should not be used together. Saxenda should also not be used in combination with another GLP-1 receptor agonist.
Hypersensitivity to liraglutide or any active ingredient.
- Saxenda must not be used as a substitute for insulin. Saxenda has not been studied in individuals taking insulin. Therefore, Saxenda and insulin should not be used together. However, if Saxenda is prescribed with insulin or insulin secretagogues (such as sulfonylureas), consider reducing the dose of insulin or sulfonylureas to reduce the risk of hypoglycaemia. Note: MedsCheck to check for clinical interactions with referral to healthcare team, provide hypoglycaemia information, assess side effect profile, refer for blood glucose monitoring.
- Saxenda is not indicated for the treatment of type 2 diabetes mellitus.
- Saxenda is not indicated in individuals with obesity secondary to endocrinological or eating disorders or in conjunction with treatment with medicinal products that may cause weight gain.
- Saxenda is not recommended in combination with other medicinal products intended for weight loss, including prescription medicines, over-the-counter medicines, and complementary medicines or herbal preparations. Note: MedsCheck to check for clinical interactions-refer to heath care professionals if appropriate.
- Cardiovascular events: An increase in heart rate with Saxenda was observed in clinical trials. For those who experience a sustained increase in resting heart rate, Saxenda should be discontinued.
- Dehydration, renal impairment, and acute renal failure: Individuals treated with Saxenda should be advised of the potential risk of dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion. In those treated with GLP-1 receptor agonists, including liraglutide, there have been reports of acute renal injury/failure and worsening of chronic renal failure. Note: MedsCheck to counsel on possible side effects and complications, referral to appropriate health care professional for review.
- Pancreatitis: Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. After initiation of Saxenda, observe carefully for signs and symptoms of pancreatitis. Do not restart Saxenda if pancreatitis is confirmed. Note: MedsCheck to counsel on side effect profile and how to manage if it happens.
- Gastrointestinal disease: Saxenda has not been studied in individuals with any form of severe gastrointestinal disease, including gastroparesis. Note: MedsCheck for side effect profile, prevention of complications, referral to appropriate allied health care team.
- Hypoglycaemia with concomitant use of diabetes therapy: The risk of serious hypoglycaemia is increased when Saxenda is used in combination with insulin secretagogues (e.g., sulfonylureas) in individuals with type 2 diabetes. The risk of hypoglycaemia can be lowered by a reduction in the dose of sulfonylurea.
- Thyroid disease: Saxenda should be used with caution in patients with thyroid disease.
- Suicide behaviour and ideation: Individuals treated with Saxenda should be monitored for the emergence of depression, suicide thoughts or behaviour, or any unusual changes in mood or behaviour. Discontinue Saxenda in those that experience suicidal thoughts or behaviours or who develop other symptoms of depression referral to appropriate allied health Note: MedsCheck for referral to appropriate health care team.
- Gastrointestinal adverse reactions: Nausea, diarrhoea, constipation, vomiting, dyspepsia, abdominal pain, dry mouth, gastritis, gastroesophageal reflux, flatulence, eructation, and abdominal distension.
Note: MedsCheck, referral to allied health professional due to side effect profile. - Hypoglycaemia in individuals with type 2 diabetes
- Thyroid C-cell tumours: Liraglutide causes thyroid C-cell tumours at clinically relevant exposures in both genders of rats and mice. It is unknown whether Saxenda causes thyroid C-cell tumours, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumours has not been determined.
Note: MedsCheck to ensure relevant referral pathways for screening. - Malignancies: In the clinical development program for weight loss, there was no imbalance for all neoplasms, combined. However, when subgroup analyses were done by individual types of cancer, imbalances were identified, including invasive breast cancer in women and colorectal neoplasms (mainly adenomas). For further information please see the full product information.
- Injection site reactions: Injection site reactions have been reported in individuals treated with Saxenda. These reactions have usually been mild and transient.
Note: MedsCheck, referral to allied health professional due to side effect profile. - Suicidal behaviour and ideation:
Note: MedsCheck side effect profile referral to GP - Cardiac disorders: Increased heart rate.</li.
- Gastrointestinal disorders. Acute pancreatitis, haemorrhagic and necrotising pancreatitis.
Note: MedsCheck, inform about side effect profile, what symptoms to be aware of for and what to do should any of these side effects occur.
The absorption of liraglutide following subcutaneous administration is slow, reaching maximum concentration approximately 11 hours post dosing.
The mean apparent volume of distribution after subcutaneous administration of a liraglutide 3.0 mg is 20-25 L (for a person weighing approximately100 kg). The mean volume of distribution after intravenous administration of liraglutide is 0.07 L/kg. Liraglutide is extensively bound to plasma protein (> 98%).
During the 24 hours following administration of a single liraglutide dose to healthy individuals, the major component in plasma was intact liraglutide. Two minor plasma metabolites were also detected.
Liraglutide is endogenously metabolised in a similar manner to large proteins without a specific organ as major route of elimination. The elimination half-life is approximately 13 hours.
For more detailed information on this product please consult the source product information.
https://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Saxenda%20pi3.pdf
For more detailed information on this product please consult the source product information.
https://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Saxenda%20pi3.p
- Please note Victoza is not PBS listed - private prescription.
- As an adjunct to healthy eating and physical activity to improve glycaemic management in individuals 10 years and older with type 2 diabetes mellitus.
- To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease.
- VICTOZA® can be injected subcutaneously in the abdomen, thigh, or upper arm once daily (at approximately the same time each day) at any time of day, without regard to meals.
- Insulin and Victoza can be injected at the same time of the day in the same region of the body but must never be mixed. Inject separately.
- Missed dose: If a dose is missed, resume the once-daily regimen as soon the dose is remembered (at the same time), Do not take an extra dose to make up for any missed doses. If more than 3 days have been missed restart VICTOZA® at 0.6 mg and titrate as per initial starting schedule to reduce chances of gastrointestinal side effects. Note: Diabetes MedsCheck for missed doses.
- Adult Dosage: Initiate VICTOZA® with a dose of 0.6 mg daily for one week to help reduce the likelihood of gastrointestinal symptoms. After one week at 0.6 mg per day, increase the dose to 1.2 mg daily. If additional glycaemic management is required, increase the dose to 1.8 mg daily after at least one week of treatment with the 1.2 mg daily dose. Note: Diabetes MedsCheck on how to use pen delivery device.
- Paediatric Dosage: Inject VICTOZA® with a dose of 0.6 mg daily. After at least one week at 0.6 mg daily, the dose may be increased to 1.2 mg daily if additional. glycaemic management is required. Further adjustment to 1.8mg after at least one week can then occur if needed.
- Victoza and Saxenda both contain the same active ingredient, liraglutide, and therefore should not be used together. Saxenda should also not be used in combination with another GLP-1 receptor agonist.
- Hypersensitivity to liraglutide or any active ingredient.
- Medullary Thyroid Carcinoma VICTOZA® is contraindicated in those with a personal or family history of medullary thyroid carcinoma (MTC) or in individuals with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Thyroid C-cell tumours: Liraglutide causes thyroid C-cell tumours at clinically relevant exposures in both genders of rats and mice. It is unknown whether Victoza causes thyroid C-cell tumours, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumours has not been determined.
Note: Diabetes MedsCheck to ensure relevant referral pathways and screening occurs. - Pancreatitis: Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. After initiation of Victoza, observe carefully for signs and symptoms. Do not restart Victoza if pancreatitis is confirmed.
Note: MedsCheck to counsel on side effect profile and how to manage if it happens. - Hypoglycaemia with concomitant use of diabetes therapy: The risk of serious hypoglycaemia is increased when Victoza is used in combination with insulin secretagogues (e.g., sulfonylureas) in individuals with type 2 diabetes. The risk of hypoglycaemia can be lowered by a reduction in the dose of sulfonylurea.
Note: Diabetes MedsCheck, counselling to ensure individual understands hypoglycaemia signs and symptoms, blood glucose monitoring, referral pathways to allied health. - Dehydration, renal impairment, and acute renal failure: Individuals treated with Victoza should be advised of the potential risk of dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion. In those treated with GLP-1 receptor agonists, including liraglutide, there have been reports of acute renal injury/failure and worsening of chronic renal failure.
Note: MedsCheck to counsel on possible side effects and complications, referral to appropriate health care professional for review. - Acute Gallbladder Disease: In the LEADER 3.1% of VICTOZA®-treated individuals versus 1.9% of placebo-treated individuals reported an acute event of gallbladder disease, such as cholelithiasis or cholecystitis. Most events required hospitalization or cholecystectomy.
Note: MedsCheck to counsel on possible side effects and complications, referral to appropriate health care professional for review.
- Risk of Thyroid C-cell Tumours
- Pancreatitis
- Hypoglycaemia
- Renal Impairment
- Hypersensitivity Reactions (especially at injection site)
- Please note Victoza is not PBS listed - private prescription.
- As an adjunct to healthy eating and physical activity to improve glycaemic management in individuals 10 years and older with type 2 diabetes mellitus.
- To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease.
- VICTOZA® can be injected subcutaneously in the abdomen, thigh, or upper arm once daily (at approximately the same time each day) at any time of day, without regard to meals.
- Insulin and Victoza can be injected at the same time of the day in the same region of the body but must never be mixed. Inject separately.
- Missed dose: If a dose is missed, resume the once-daily regimen as soon the dose is remembered (at the same time), Do not take an extra dose to make up for any missed doses. If more than 3 days have been missed restart VICTOZA® at 0.6 mg and titrate as per initial starting schedule to reduce chances of gastrointestinal side effects. Note: Diabetes MedsCheck for missed doses.
- Adult Dosage: Initiate VICTOZA® with a dose of 0.6 mg daily for one week to help reduce the likelihood of gastrointestinal symptoms. After one week at 0.6 mg per day, increase the dose to 1.2 mg daily. If additional glycaemic management is required, increase the dose to 1.8 mg daily after at least one week of treatment with the 1.2 mg daily dose. Note: Diabetes MedsCheck on how to use pen delivery device.
- Paediatric Dosage: Inject VICTOZA® with a dose of 0.6 mg daily. After at least one week at 0.6 mg daily, the dose may be increased to 1.2 mg daily if additional. glycaemic management is required. Further adjustment to 1.8mg after at least one week can then occur if needed.
- Victoza and Saxenda both contain the same active ingredient, liraglutide, and therefore should not be used together. Saxenda should also not be used in combination with another GLP-1 receptor agonist.
- Hypersensitivity to liraglutide or any active ingredient.
- Medullary Thyroid Carcinoma VICTOZA® is contraindicated in those with a personal or family history of medullary thyroid carcinoma (MTC) or in individuals with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Thyroid C-cell tumours: Liraglutide causes thyroid C-cell tumours at clinically relevant exposures in both genders of rats and mice. It is unknown whether Victoza causes thyroid C-cell tumours, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of liraglutide-induced rodent thyroid C-cell tumours has not been determined.
Note: Diabetes MedsCheck to ensure relevant referral pathways and screening occurs. - Pancreatitis: Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. After initiation of Victoza, observe carefully for signs and symptoms. Do not restart Victoza if pancreatitis is confirmed.
Note: MedsCheck to counsel on side effect profile and how to manage if it happens. - Hypoglycaemia with concomitant use of diabetes therapy: The risk of serious hypoglycaemia is increased when Victoza is used in combination with insulin secretagogues (e.g., sulfonylureas) in individuals with type 2 diabetes. The risk of hypoglycaemia can be lowered by a reduction in the dose of sulfonylurea.
Note: Diabetes MedsCheck, counselling to ensure individual understands hypoglycaemia signs and symptoms, blood glucose monitoring, referral pathways to allied health. - Dehydration, renal impairment, and acute renal failure: Individuals treated with Victoza should be advised of the potential risk of dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion. In those treated with GLP-1 receptor agonists, including liraglutide, there have been reports of acute renal injury/failure and worsening of chronic renal failure.
Note: MedsCheck to counsel on possible side effects and complications, referral to appropriate health care professional for review. - Acute Gallbladder Disease: In the LEADER 3.1% of VICTOZA®-treated individuals versus 1.9% of placebo-treated individuals reported an acute event of gallbladder disease, such as cholelithiasis or cholecystitis. Most events required hospitalization or cholecystectomy.
Note: MedsCheck to counsel on possible side effects and complications, referral to appropriate health care professional for review.
- Risk of Thyroid C-cell Tumours
- Pancreatitis
- Hypoglycaemia
- Renal Impairment
- Hypersensitivity Reactions (especially at injection site)
- The results of bioequivalence studies in healthy subjects demonstrated that KOMBIGLYZE XRcombination tablets are bioequivalent to coadministration of corresponding doses of saxagliptin and metformin hydrochloride modified release as individual tablets.
- The following statements reflect the pharmacokinetic properties of the individual active substancesof KOMBIGLYZE XR.
Saxagliptin: The pharmacokinetics of saxagliptin have been extensively characterised in healthy subjects andpeople with type 2 diabetes. Saxagliptin was rapidly absorbed after oral administration, withmaximum saxagliptin plasma concentrations (Cmax) usually attained within two hours afteradministration in the fasted state. Following a single oral dose of 5 mg saxagliptin to healthy subjects, the mean plasma terminal half-life (t1/2) for saxagliptin was 2.5 hours, and the mean t1/2 value for plasma DPP-4 inhibition was26.9 hours. The inhibition of plasma DPP-4 activity by saxagliptin for at least 24 hours after oraladministration is due to high potency, high affinity, and extended binding to the active site. Appreciable accumulation was observed with repeated once-daily dosing at any dose level. Nodose- and time-dependence was observed in the clearance of saxagliptin and its major metaboliteover 14 days of once-daily dosing with saxagliptin at doses ranging from 2.5 mg to 400 mg.
Metformin hydrochloride: Metformin extended release Cmax is achieved with a median value of 7 hours. The extent ofmetformin absorption from the metformin extended-release tablet is increased by approximately50% when given with food. At steady state, the AUC and Cmax are less than dose proportional formetformin extended release within the range of 500 to 2000 mg. After repeated administration ofmetformin extended-release, metformin did not accumulate in plasma. Metformin is excretedunchanged in the urine and does not undergo hepatic metabolism.
- Saxagliptin: Based on food effects studies, saxagliptin may be administered with or without food. However, inpivotal efficacy and safety studies saxagliptin was generally taken prior to the morning meal. Theamount of saxagliptin absorbed following an oral dose is at least 75%. The absolute oralbioavailability of saxagliptin is approximately 50% (90% CI of 48-53%). Food had relativelymodest effects on the pharmacokinetics of saxagliptin in healthy subjects.
- Metformin hydrochloride: Following a single oral dose of metformin extended release, Cmax is achieved with a median valueof 7 hours and a range of 4 to 8 hours. Both high and low-fat mealshad the same effect on the pharmacokinetics of metformin extended release.
- Saxagliptin: The in vitro protein binding of saxagliptin and its major metabolite in human serum is belowmeasurable levels. Thus, changes in blood protein levels in various disease states (e.g., renal orhepatic impairment) are not expected to alter the disposition of saxagliptin.
- Metformin hydrochloride: Distribution studies with extended-release metformin have not been conducted; however, theapparent volume of distribution (V/F) of metformin following single oral doses of immediaterelease metformin 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasmaproteins, in contrast to sulfonylureas, which are more than 90% protein bound.
- Saxagliptin: The metabolism of saxagliptin is primarily mediated by cytochrome P450 3A4/5 (CYP3A4/5). Themajor metabolite of saxagliptin is also a reversible, competitive DPP-4 inhibitor, half as potent assaxagliptin. It also demonstrates selectivity for DPP-4 versus other DPP enzymes, with greater than163-fold selectivity over DPP-8 and DPP-9.
- Metformin hydrochloride: Intravenous single-dose studies in normal subjects demonstrate that metformin is excretedunchanged in the urine and does not undergo hepatic metabolism (no metabolites have beenidentified in humans) or biliary excretion.
- Saxagliptin: Saxagliptin is eliminated by both renal and hepatic pathways. The average renal clearance of saxagliptin (~230mL/min) was greater than the average estimated glomerular filtration rate (~120 mL/min),suggesting some active renal excretion. For the major metabolite, renal clearance values werecomparable to estimated glomerular filtration rate. A total of 22% of the administered radioactivitywas recovered in faeces representing the fraction of the saxagliptin dose excreted in bile and/orunabsorbed drug from the gastrointestinal tract.
- Metformin hydrochloride: Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates thattubular secretion is the major route of metformin elimination. Following oral administration,approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours. When renal function is impaired, renal clearance is decreased in proportion to that ofcreatinine and thus the elimination half-life is prolonged, leading to increased levels of metforminin plasma.
About Our Experts
Family-owned and operated company
specializing in flooring solutions
Lorem ipsum dolor sit amet, te has solet postea. Voluptua quaestio dissentias has ex, no interpretaris, viderer pertinax repudiandae ne ius, qui ne porro insolens instructior. Graece euripidis instructior an vix, eum et equidem expetenda concludaturque, ut est Ex est dicit graeco consequat, mel rebum placerat et. Facer recusabo reprehendunt vel at. Delenit repudiare in mei, mazim assentior voluptaria et pri. Ad tempor tritani qui, et elitr consequat
Like many health conditions, diabetes is often misunderstood and there is a lot of misinformation so increasing the coordination amongst pharmacist and CDE to provide support and knowledge to people living with diabetes can be very powerful.
Meet The Experts
Fully-licensed and insured flooring experts
with over 20 years of experience
Dr Alan Barclay
Kirrily Chambers
Cindy Tolba
Frequently Asked Questions
Here are some answers to the questions we receive the most about our services.
If we missed anything, please do not hesitate to contact us. We’ll be happy to help
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
About Our Experts
Family-owned and operated company
specializing in flooring solutions

Lorem ipsum dolor sit amet, te has solet postea. Voluptua quaestio dissentias has ex, no interpretaris, viderer pertinax repudiandae ne ius, qui ne porro insolens instructior. Graece euripidis instructior an vix, eum et equidem expetenda concludaturque, ut est Ex est dicit graeco consequat, mel rebum placerat et. Facer recusabo reprehendunt vel at. Delenit repudiare in mei, mazim assentior voluptaria et pri. Ad tempor tritani qui, et elitr consequat
Meet The Experts
Passionate about coaching healthcare professionals and empowering people living with diabetes
Dr Alan Barclay
Kirrily Chambers
Cindy Tolba
Frequently Asked Questions
Here are some answers to the questions we receive the most about our services.
If we missed anything, please do not hesitate to contact us. We’ll be happy to help
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Like many health conditions, diabetes is often misunderstood and there is a lot of misinformation so increasing the coordination amongst pharmacist and CDE to provide support and knowledge to people living with diabetes can be very powerful.
About Our Experts
Family-owned and operated company
specializing in flooring solutions

Lorem ipsum dolor sit amet, te has solet postea. Voluptua quaestio dissentias has ex, no interpretaris, viderer pertinax repudiandae ne ius, qui ne porro insolens instructior. Graece euripidis instructior an vix, eum et equidem expetenda concludaturque, ut est Ex est dicit graeco consequat, mel rebum placerat et. Facer recusabo reprehendunt vel at. Delenit repudiare in mei, mazim assentior voluptaria et pri. Ad tempor tritani qui, et elitr consequat
Meet The Expert Coaches
Passionate about coaching healthcare professionals and empowering people living with diabetes
Dr Alan Barclay
Kirrily Chambers
Cindy Tolba
Frequently Asked Questions
Here are some answers to the questions we receive the most about our services.
If we missed anything, please do not hesitate to contact us. We’ll be happy to help
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Like many health conditions, diabetes is often misunderstood and there is a lot of misinformation so increasing the coordination amongst pharmacist and CDE to provide support and knowledge to people living with diabetes can be very powerful.
About Our Experts
Family-owned and operated company
specializing in flooring solutions

Lorem ipsum dolor sit amet, te has solet postea. Voluptua quaestio dissentias has ex, no interpretaris, viderer pertinax repudiandae ne ius, qui ne porro insolens instructior. Graece euripidis instructior an vix, eum et equidem expetenda concludaturque, ut est Ex est dicit graeco consequat, mel rebum placerat et. Facer recusabo reprehendunt vel at. Delenit repudiare in mei, mazim assentior voluptaria et pri. Ad tempor tritani qui, et elitr consequat
Meet The Expert Coaches
Passionate about coaching healthcare professionals and empowering people living with diabetes
Dr Alan Barclay
Kirrily Chambers
Cindy Tolba
Frequently Asked Questions
Here are some answers to the questions we receive the most about our services.
If we missed anything, please do not hesitate to contact us. We’ll be happy to help
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Like many health conditions, diabetes is often misunderstood and there is a lot of misinformation so increasing the coordination amongst pharmacist and CDE to provide support and knowledge to people living with diabetes can be very powerful.
About Our Experts
Family-owned and operated company
specializing in flooring solutions

Lorem ipsum dolor sit amet, te has solet postea. Voluptua quaestio dissentias has ex, no interpretaris, viderer pertinax repudiandae ne ius, qui ne porro insolens instructior. Graece euripidis instructior an vix, eum et equidem expetenda concludaturque, ut est Ex est dicit graeco consequat, mel rebum placerat et. Facer recusabo reprehendunt vel at. Delenit repudiare in mei, mazim assentior voluptaria et pri. Ad tempor tritani qui, et elitr consequat
Meet The Expert Coaches
Passionate about coaching healthcare professionals and empowering people living with diabetes
Dr Alan Barclay
Kirrily Chambers
Cindy Tolba
Frequently Asked Questions
Here are some answers to the questions we receive the most about our services.
If we missed anything, please do not hesitate to contact us. We’ll be happy to help
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Like many health conditions, diabetes is often misunderstood and there is a lot of misinformation so increasing the coordination amongst pharmacist and CDE to provide support and knowledge to people living with diabetes can be very powerful.
About Our Experts
Family-owned and operated company
specializing in flooring solutions

Lorem ipsum dolor sit amet, te has solet postea. Voluptua quaestio dissentias has ex, no interpretaris, viderer pertinax repudiandae ne ius, qui ne porro insolens instructior. Graece euripidis instructior an vix, eum et equidem expetenda concludaturque, ut est Ex est dicit graeco consequat, mel rebum placerat et. Facer recusabo reprehendunt vel at. Delenit repudiare in mei, mazim assentior voluptaria et pri. Ad tempor tritani qui, et elitr consequat
Meet The Expert Coaches
Passionate about coaching healthcare professionals and empowering people living with diabetes
Dr Alan Barclay
Kirrily Chambers
Cindy Tolba
Frequently Asked Questions
Here are some answers to the questions we receive the most about our services.
If we missed anything, please do not hesitate to contact us. We’ll be happy to help
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Lorem ipsum dolor sit amet consectet adipisc elit?
Like many health conditions, diabetes is often misunderstood and there is a lot of misinformation so increasing the coordination amongst pharmacist and CDE to provide support and knowledge to people living with diabetes can be very powerful.